Introduction
A fuel cell is a Energy generating device that generates electricity from chemical energy. A hydrogen fuel cell is a device that uses hydrogen (or hydrogen-rich Fuel) and oxygen to create electricity. The efficiency of a fuel cell is potentially higher than the of combustion engines. Fuel cells are similar to batteries but with replenishable material (Fuel). If the pure form of hydrogen is used as a fuel in a hydrogen fuel cell, fuel cells emit only water and heat, eliminating concerns about air pollutants or greenhouse gases. Under consideration for large-scale power generation and small-scale portable applications, the fuel cell is suitable.
History of Fuel Cells
- 1839 Sir William Grove – credited with first electrochemical H2 /O2 reaction to create energy
- 1950s Thomas Bacon – fuel cell stack
- 1950s-now – fuel cells in space program (alkaline)
- 1960s other fuel cells discovered – phosphoric acid, SOFC, molten carbonate, PEMFC
- 1970s – first stationary power applications
- 1990s-now – transportation and other applications under development
- 2006 – commercial sales for communications backup power system
Fuel Cell Classification:
- Polymer Electrolyte Membrane Fuel Cell (PEMFC)
- Alkaline Fuel Cell (AFC)
- Phosphoric Acid Fuel Cell (PAFC)
- Molten Carbonate Fuel Cell (MCFC)
- Solid Oxide Fuel Cell (SOFC)
In terms of Fuel Used:
- Hydrogen Fuel Cell
- Alcohol Fuel Cell
- Carbon Monoxide
- Methane
In terms of operating temperature range:
- High-temperature Fuel cell
- Low-temperature Fuel cell
 |
| source: https://americanhistory.si.edu/fuelcells/basics.htm |
Components of Fuel Cell
The most common type of fuel cell is the polymer electrolyte membrane (PEM) fuel cell. This type of fuel cell consists of an electrolyte membrane sandwiched between a cathode (positive electrode) and an anode (negative electrode).
Membrane
The membrane between anode and cathode allows the positively charged protons (H+) to pass through to the cathode but restrict the negatively charged electrons(e-).
The negatively charged electrons should flow around the membrane through an external circuit generating an electrical current.
Properties desired for membrane electrolyte:
- High ionic conductivity (minimum resistive losses)
- Low electronic conductivity (minimizes current losses)
- Chemical stability both in oxidizing (anode) and reducing (cathode)
- Low fuel crossover
- Mechanical strength manufacturability
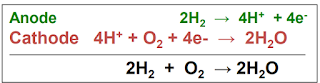 |
fig: Hydrogen fuel cell reaction |
Cathode
The cathode is the positive electrode at which a gain of electrons (reduction) occurs. In a fuel cell, the cathode is considered electrically positive. The cathode is prepared by adding uniform platinum particles supported on carbon particles, which act as catalysts, increasing the reduction process rate. The cathode is made porous so that oxygen can pass through it.
Anode
An anode is an electrode at which there is loss of electrons takes place (oxidation). In a fuel cell, the anode is considered electrically negative. The anode is prepared by adding uniform platinum particles supported on carbon particles, which act as a catalyst, increasing the rate of the oxidation process. The anode electrode is porous so that hydrogen can pass through it.







0 Comments