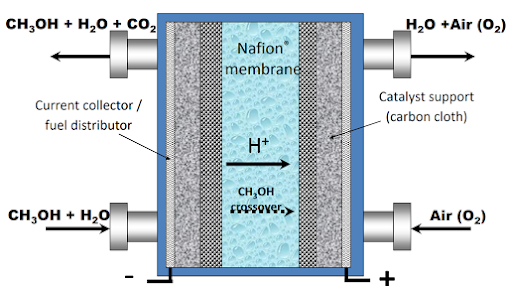
Direct Methanol Fuel Cell
DMFC uses Methanol and water as the fuel on the anode side and Air in cathode side. DMFC is generally seen as the most viable alternative to LI-ion batteries. This is because it has less ancillary and is more simplified than that of H2 PEFC; additionally, it uses liquid fuel, which is easy to store and transport.
DMFC can compete with advanced Li-ion batteries in terms of gravimetric density of 120 -160Wh/kg, Volumetric density of 230-270 Wh/l.
For the DMFC, both anode and cathode activation polarization is significant. However, reduced performance compared to the H2 PEFC is tolerable in light of other advantages of the DMFC, namely:
- DMFC doesn't require a separate cooling or humidification subsystem Because the anode flow is mostly liquid (gaseous CO2 is a product of methanol oxidation).
- Liquid fuel used in the anode results in lower parasitic pumping requirements compared to gas flow. Many passive DMFC designs operate without any external parasitic losses instead of relying on natural forces such as capillary action, buoyancy, and diffusion to deliver reactants.
- The highly dense liquid fuel stored at ambient pressure eliminates problems with fuel storage volume. With highly concentrated Methanol as fuel (>10 M), passive DMFC system power densities can compare favorably to advanced Li-ion batteries.
- A Reformer system, like in a hydrogen fuel cell, is not required.
- DMFC uses liquid fuel, so easy to store and transport
 |
fig: Direct methanol fuel cell and associated mass transfer processes. (Courtesy of K. Sharp, Penn State University |
The DMFC solves many problems associated with the hydrogen PEFC system; Technical issue and challenges of DMFC are summarized below:
1. Water Management
Even though external humidification is not needed in the DMFC, prevention of cathode flooding is critical to ensure adequate performance.
2. Methanol Crossover
There is a high crossover rate of Methanol from anode to cathode because of the high concentration of Methanol at the anode side. This results in a mixed potential at the cathode from crossover methanol oxidation and dramatically reduces the open-circuit voltage of the DMFC from the theoretical value of ∼1.2 V to around 0.7–0.8 V.
To combat the methanol crossover, several approaches have been used
- Use of dilute methanol solution : low molarity solution of methanol can reduce crossover of the fuel.
- Use of thicker Electrolyte: It can reduce the crossover of methanol but also limits the performance of fuel cell
- Diffusion barrier on anode: Diffusion barrier is the alternative solution of thicker layer
- Capillary pressure management: Highly concentrated methanol can be used.
3. Catalyst poisoning
This problem arise due to the strong CO adsorption on the platinum surface(COads), which leads to the poisoning of active sites for hydrogen oxidation reaction. The bond between Pt-CO is very strong and difficult to oxidize and performance gets retarded.
 |
| fig: formation of CO on Pt |
To Remove the COads from the surface it is necessary to generate some oxygenated species that can react with C)ads producing CO2 and release some free sites on Pt surface for hydrogen oxidation reaction.
To overcome the catalyst poisoning Bi functional catalyst is used. More exploited Bi-functional catalyst is PT-Ru Bi-functional catalyst. It adsorb OH or H2O and During the formation of the metallic bond between Bi- functional catalyst it weaken the bond between the Pt-CO.
 |
| fig: Bi-Functional Catalyst |
4. Counter flow and Removal of Carbon Dioxide is produced at the anode surface, resulting in countercurrent two-phase flow in the anode DM that can block access to the catalyst layer.
5. Methanol Safety
Methanol is slightly toxic, spreads more easily into the ground than gasoline, and is highly flammable and miscible in water, so that contamination with reservoirs is very simple







0 Comments